Table of contents
Updated - June 3, 2025
The shorter the PEG chain (polyethylene glycol), the higher the toxicity of the LNP. BioNTech explains this in its publication "Non-Immunotherapy Application of LNP-mRNA: Maximizing Efficacy and Safety" of 10.05.2021, albeit formulated - somewhat - differently: "Maximizing Efficacy".
The toxicity is based on the fact that all three of the ionizable cationic lipids are not biodegradable and their inevitable accumulation in the body has cytotoxic consequences.
Which LNPs were used?
Pfizer / BioNTech - BNT162b2 - ALC-0315 (ionizable) and ALC-0159 (PEGylated)
Moderna – SM-102 (ionizable) and PEG2000-DMG (PEGylated)
For all the lipids mentioned above, the manufacturers (e.g. Echelon Biosciences) "... This is a reagent grade product, for research use only."
Toxicity has not been verified by studies
According to the Publication "Nonclinical Evaluation Report BNT 162b2 mRNA COVID-19 vaccine COMIRNATY™" of the Australian Government - Department of Health from January 2021 can be read on page 11: "The toxicity of LNP formulation or the novel excipients alone was not specifically studied."
In other words, no toxicity studies have been conducted on the use and effects of the new LNP excipients mentioned above.
Accumulation in the liver
Many drugs are partially or completely metabolized by the liver and enter the bloodstream. The above-mentioned excipient ALC-315 also accumulates in the liver, as can be seen from the Partial answer to question no. 2021-4379 under the Freedom of Information Act (FOIA), which is the subject of the complaint in Judicial Watch, Inc. v. U.S. Department of Health and Human Services (21-cv-2418), currently pending in the U.S. District Court for the District of Columbia, at page 16 of 466:
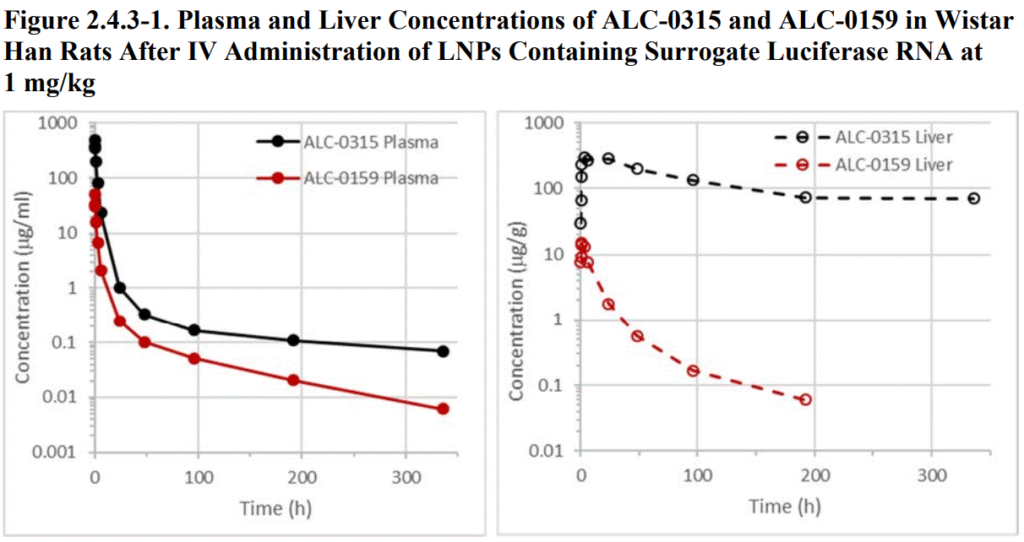
Also mentioned on page 45 of the above. Reports:

On page 46, the purely theoretically assumed metabolic pathway is outlined on the basis of in vitro experiments, although this has not been verified in humans.
While Moderna parted company with ALC-0315 due to liver incompatibility and developed the SM platform itself (ALC-0315 had proven to be too toxic for gene therapy of the liver disease Crigler-Najjar syndrome, so that the then research partner Alexion stopped the study for safety reasons), BioNTech / Pfizer continues to use them against its better judgment.
The path into the liver and the length of the chain
… to be continued …